The drug Aducanumab could be approved in the US and EU soon. It effectively combats the amyloid beta plaques in the brain that are indicative of Alzheimer’s disease.
But does it also slow down memory loss?

Great hopes in Alzheimer’s research are currently pinned on the drug aducanumab. The US Food and Drug Administration (FDA) could decide on approval on June 7, and the European Medicines Agency (EMA) by the end of the year.
The drug has shown the ability to effectively remove harmful amyloid beta plaques (known as A-beta for short) from the brain. But debate continues in the scientific community: Is this also enough to stop memory decline?
A dilemma: Patients would have to take the drug long before they show the first symptoms of dementia.
A look at the healthy, not the sick
The inventors of the drug come from the University of Zurich. Roger Nitsch and Christoph Hock did not primarily look at Alzheimer’s patients, but at healthy and fit people of advanced age. They looked specifically for immune cells able to form antibodies against A-beta. And they found what they were looking for.
In painstaking work, they decoded the antibodies and reconstructed them in the lab. Together with the US company Biogen, they then brought the active substance into clinical trials.
Like a waterfall
Alzheimer’s researchers now largely agree that Alzheimer’s disease takes the form of a very long-lasting cascade in which various deterioration processes of brain cells follow one another. Central to this is the formation of amyloid beta plaques. The protein occurs in all humans as a monomer (simple molecule). However, when it combines to form oligomers (a precursor of plaques), it becomes harmful. Next comes the activation of cellular immune defenses and the subsequent formation of more deposits, the tau plaques.
Researchers also see the biggest problem in this cascade: Only if it is possible to combat it at an early stage can memory loss possibly be stopped in the long term.
Christian Haas, professor of molecular neurodegeneration at the German Center for Neurodegenerative Diseases (DZNE) in Munich, compares the progression of the disease to waterfalls, in an interview with DW: “If we want to turn off the water at the top, we have to do it directly with the amyloid. If we go in too late and too much of the amyloid has accumulated, the tau protein alone can keep the waterfall going.”
So the success of a treatment depends on how early doctors detect the disease and whether they manage to intervene with the right agent. The problem is that Alzheimer’s usually goes unnoticed for a long time, and by the time memory loss becomes apparent, it is already too late. This problem was also reflected in the clinical trials of aducanumab.
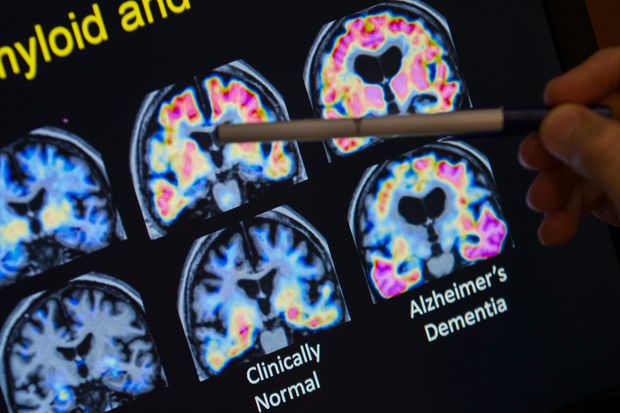
Up and down in the clinical trials
Three clinical trials, named Prime, Emerge, and Engage, had been conducted by Biogen before the company submitted the compound to the FDA for approval in 2019 and to the EMA in 2020. The researchers’ assessment of the study results kept changing; it was like a roller coaster ride.
In the Prime trial, aducanumab had successfully targeted A beta plaques in 166 Alzheimer’s patients in 2016. However, in the subsequent Emerge and Engage studies with a total of 3,200 participants, there were conflicting assessments of the results. In March 2019, both studies were halted because interim results showed no cognitive improvements in the patients. “And in the end, that’s all that really matters,” Christian Haas said.
But in October 2019, the researchers changed their assessment. There had been a demonstrable positive effect on memory performance in patients in the Emerge study. Biogen then applied to the FDA for approval.
Shortly thereafter, however, an independent panel of experts commissioned by the FDA again confirmed that the drug was of little benefit. This contradicted an initial assessment by the public health agency. As a result, it extended the review process for the drug until June 2021.
A lot helps a lot ― or does it also do harm?
The different assessments of the efficacy of aducanumab was also related to the dosage of the drug that participants received in the various studies. The physicians were able to determine the strongest effect in the test subjects who had received a particularly high dosage. In them, the decline in cognitive performance seemed to slow down. Read more from DW