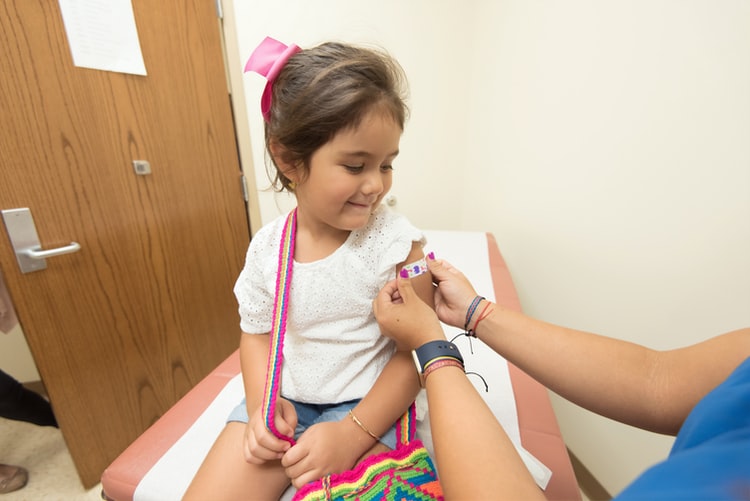
Clinical trials are underway to test the safety and effectiveness of a Covid vaccine for children as young as six months

Written content from Jerry Carnes
COVID-19 vaccines will soon be available to anyone in Georgia 16 and older, while younger children continue to wait for a vaccine.

Clinical trials are underway to test the safety and effectiveness of vaccines on children. Moderna announced a trial for children under the age of 12 and as young as six months.
Trials on the vaccines currently available did not include children under 16.
Here’s why.
Dr. M.G. Finn of Georgia Tech’s School of Chemistry tells us children and adults differ in more than size.
“Children and adults are physiologically different,” said Dr. Finn. “The biology is different. Kids are not little adults.”

Adults face the highest risk of contracting and suffering severe cases of COVID-19 so initial vaccine trials focused on them.
“Vaccine trials are both expensive, very large scale, and for children, you need to be even extra careful,” Finn explained. “To get the vaccines done in the accelerated time frame they were done — it didn’t make sense at the time to test children.”

Clinical trials to test vaccines for children are different.
“The numbers are different, the mechanics are different, the medical hazards and rewards are different,” said Finn.
According to the American Academy of Pediatrics, there were 3,341,608 COVID-19 cases involving children as of March 18, or approximately 13% of all cases in the United States. Read more from 11Alive